The PFAS Metabolic Reaction Library has been developed as a component of the Chemical Transformation Simulator (CTS), a web-based software tool under development in EPA’s Office of Research and Development. The library is implemented in CTS to predict the likely metabolic products of per- and polyfluoroalkyl substances (PFAS).
Version 1.1 of the PFAS Metabolic Reaction Library contains 76 reaction schemes:
Conjugation Schemes
Conjugation: Glucuronide-fluorotelomer alcohol conjugate formation
Conjugation: Glucuronide-fluorotelomer sec-alcohol conjugate formation
Conjugation: Glutathione-epoxide conjugate formation
Conjugation: Glutathione-ether conjugate formation
Conjugation: Glutathione-unsaturated fluorotelomer acid conjugate formation
Conjugation: Glutathione-unsaturated fluorotelomer aldehyde conjugate formation
Conjugation: Glutathione-vinyl ether conjugate formation
Conjugation: Hydrolysis of glutathione-ether conjugate to cysteine-ether conjugate_PTP
Conjugation: Hydrolysis of glutathione-vinyl ether conjugate to cysteine-vinyl ether conjugate_PTP
Conjugation: Hydrolysis of sulfide conjugate to carboxylic acid
Conjugation: Hydrolysis of vinyl sulfide conjugate to carboxylic acid
Conjugation: Reduction of alpha-keto glutathione conjugate to alpha-hydroxy glutathione conjugate
Conjugation: S-Dealkylation of cysteine-ether conjugate to beta thio ether_PTP
Conjugation: S-Dealkylation of cysteine-vinyl ether conjugate to beta thio vinyl ether_PTP
Conjugation: Sulfate-fluorotelomer alcohol conjugate formation
Conjugation: Taurine-fluorotelomer acid conjugate formation
Decarboxylation Schemes
Decarboxylation: Alpha hydroxy fluorotelomer carboxylic acid to fluorotelomer aldehyde
Decarboxylation: Beta carboxy ketone to methyl ketone
Epoxidation Scheme
Epoxidation: Alkene to epoxide_PTP
Hydrolysis Schemes
Hydrolysis: Acid fluoride to carboxylic acid
Hydrolysis: Alpha difluoro alcohol to acid fluoride
Hydrolysis: Alpha fluoro secondary alcohol to ketone
Hydrolysis: Amide to carboxylic acid
Hydrolysis: Carboxylic acid ester to carboxylic acid
Hydrolysis: Diperfluorophosphinate to perfluorophosphonate
Hydrolysis: Diphosphate ester to monophosphate ester
Hydrolysis: Epoxide to diol_PTP
Hydrolysis: Fluorotelomer acid to unsaturated telomer acid
Hydrolysis: Fluorotelomer aldehyde to fluorotelomer unsaturated aldehyde
Hydrolysis: Fluorotelomer iodide to fluorotelomer alcohol
Hydrolysis: Monophosphate ester to alcohol
Hydrolysis: Perfluorinated epoxide to alpha-keto carboxylic acid
Hydrolysis: Sulfonamide to sulfonic acid
Hydrolysis: Sulfonyl fluoride to sulfonic acid
Hydroxylation Schemes
Hydroxylation: Fluorotelomer acid to alpha-hydroxy fluorotelomer acid_PTP
Hydroxylation: Unsaturated Fluorotelomer acid to beta-hydroxy unsaturated fluorotelomer acid_PTP
N-Deacetylation Schemes
N-Deacetylation: N-acetyl N-alkyl sulfonamide to N-alkyl sulfonamide
N-Deacetylation: N-acetyl sulfonamide to sulfonamide
N-Dealkylation Schemes
N-Dealkylation: N-alkyl sulfonamide to sulfonamide
N-Demethylation Schemes
O-Demethylation Schemes
O-Demethylation: Alpha-difluoro methyl ether to alcohol
O-Demethylation: Fluoromethyl ether to secondary alcohol
Oxidation Schemes
Oxidation: Beta-H unsaturated fluorotelomer aldehyde to beta-H unsaturated fluorotelomer carboxylic acid
Oxidation: Beta-keto aldehyde to beta-keto acid_PTP
Oxidation: Beta oxidation of beta keto fluorotelomer acid_PTP
Oxidation: Fluorotelomer alcohol to fluorotelomer aldehyde
Oxidation: Fluorotelomer alcohol to fluorotelomer carboxylic acid with loss of CF2 and methyl groups
Oxidation: Fluorotelomer alcohol to fluorotelomer carboxylic acid with loss of methyl group
Oxidation: Fluorotelomer aldehyde to fluorotelomer carboxylic acid
Oxidation: Fluorotelomer carboxylic acid to 2,3-unsaturated fluorotelomer carboxylic acid
Oxidation: Fluorotelomer iodide to alpha-beta unsaturated fluorotelomer iodide_PTP
Oxidation: Fluorotelomer unsaturated aldehyde to fluorotelomer unsaturated carboxylic acid
Oxidation: N-Alkyl sulfonamide alcohol to N-alkyl sulfonamide carboxylic acid
Oxidation: X:3 fluorotelomer aldehyde to X:3 fluorotelomer carboxylic acid
Reduction Schemes
Reduction: 2,3-Unsaturated fluorotelomer carboxylic acid to fluorotelomer carboxylic acid
Reduction: Beta-F fluorotelomer unsaturated acid to beta-H fluorotelomer unsaturated acid
Reduction: Beta-F fluorotelomer unsaturated aldehyde to beta-H fluorotelomer unsaturated aldehyde
Reduction: Beta-H fluorotelomer unsaturated aldehyde to fluorotelomer aldehyde
Reduction: Hydrogenolysis of chlorinated perfluorinated ether
Reduction: Methyl ketone to alcohol
Reduction: Perfluoroalkyl sulfonyl fluoride to perfluoroalkyl sulfinic acid
Tautomerization Scheme
Tautomerization: Beta hydroxy unsaturated fluorotelomer acid to beta keto fluorotelomer acid
Tautomerization: Beta hydroxy unsaturated fluorotelomer aldehyde to beta keto fluorotelomer aldehyde
The reaction schemes are written as generic reaction equations defining how a particular structural fragment will be modified by the transformation reaction. These schemes are not balanced reactions showing all reactants and products (e.g., H2O, OH- and/or H+ are not shown as reactants in the schemes). Additionally, the structural fragments in the reaction schemes are written with a minimal amount of specificity. For example, the inclusion of hydrogen atoms in the scheme implies that there is a requirement for a hydrogen atom to present be in the specified position for the reactions to proceed; otherwise, it is assumed that, for simplicity, hydrogen atoms are not explicitly included.
The schemes are encoded using the notation and structural query features from ChemAxon’s Marvin tools. Definitions of some common symbols used in the reaction schemes are provided below:
· L[a1;a2;…] is a list of possible atoms (a1, a2, …) that can occupy the position within the fragment
· (A) is used to indicate an aliphatic carbon atom
· (a) is used to indicate an aromatic carbon atom
· (H1) indicates that the atom is bonded to one hydrogen
· (s*) indicates substituent count is as drawn for the atom
· AH is used to represent any atom including hydrogen
Examples are provided for each reaction scheme in the library. As is the case for the reaction schemes themselves, the example reactions do not show all of the reactants and products involved in the reduction reaction. The example chemical is shown as the only reactant, and the products are the major transformation products reported in the study. These example transformations from the peer-reviewed literature and government regulatory reports were used to test the reaction schemes in the library.
The schemes within the Anaerobic Biotransformation Reaction Library are ranked on a scale of one to seven according to their relative rate of transformation, with a higher rank indicating a faster transformation rate. A database of measured rate constants or half-lives was compiled from a survey of peer-reviewed scientific literature and reports by government regulatory agencies to assign these ranks to each reaction scheme.
Conjugation Schemes
SCHEME:

EXAMPLES:
· 8:2 S-Cys-unsaturated fluorotelomer carboxylic acid (8:2 FTUCA-SCys; DTXSID901348628) (Fasano et al., 2009)

SCHEME:

EXAMPLES:
· 6:2 S-Cys-unsaturated fluorotelomer alcohol (6:2 uFTOH-SCys; DTXSID601348631) (Zhang et al., 2020)

· 8:2 S-Cys-unsaturated fluorotelomer alcohol (8:2 uFTOH-SCys; DTXSID901348616 ) (Fasano et al, 2009; Zhang et al., 2016)

Conjugation: Glucuronide-fluorotelomer alcohol conjugate formation
SCHEME:

EXAMPLES:
· 8:2 Fluorotelomer alcohol (8:2 FTOH; DTXSID7029904) (Fasano et al., 2009; Nabb et al., 2007; Martin et al., 2005)

Conjugation: Glucuronide-fluorotelomer sec-alcohol conjugate formation
SCHEME:

EXAMPLES:
· 7:2 secondary fluorotelomer alcohol (7:2 sFTOH; DTXSID10517598) (Fasano et al., 2009; Nabb et al., 2007)

Conjugation: Glutathione-epoxide conjugate formation
SCHEME:

EXAMPLES:
· 2-fluoro-2-(trifluoromethyl)oxirane (Schuster et al., 2010)

Conjugation: Glutathione-ether conjugate formation
SCHEME:

EXAMPLES:
· 1,1,3,3,3-pentafluoro-2-(fluoromethoxy)prop-1-ene (DTXSID10973690) (Jin et al., 1996)

Conjugation: Glutathione-unsaturated fluorotelomer acid conjugate formation
SCHEME:

EXAMPLES:
· 8:2 Fluorotelomer unsaturated carboxylic acid (8:2 FTUCA, DTXSID60825615) (Butt et al., 2010a; Fasano et al., 2009; Martin et al., 2005; Nabb et al., 2007; Zhang et al., 2016)

Conjugation: Glutathione-unsaturated fluorotelomer aldehyde conjugate formation
SCHEME:

EXAMPLES:
· 8:2 Fluorotelomer unsaturated aldehyde (8:2 FTUAL, DTXSID701026622) (Butt et al., 2010a; Fasano et al., 2009; Martin et al., 2005; Nabb et al., 2007; Zhang et al., 2016)

Conjugation: Glutathione-vinyl ether conjugate formation
SCHEME:

EXAMPLES:
· 1,1,3,3,3-pentafluoro-2-(fluoromethoxy)prop-1-ene (DTXSID10973690) (Jin et al., 1996)

Conjugation: Hydrolysis of glutathione-ether conjugate to cysteine-ether conjugate_PTP
SCHEME:

EXAMPLES:
· 2-amino-4-{[1-(carboxymethylcarbamoyl)-2-{[1,1,3,3,3-pentafluoro-2-(fluoromethoxy)propyl]sulfanyl}ethyl]carbamoyl}butanoic acid (Jin et al., 1995)

SCHEME:

EXAMPLES:
· Glutathione-unsaturated fluorotelomer acid conjugate (DTXSID101348636) (Fasano et al., 2009; Zhang et al., 2016)

SCHEME:

EXAMPLES:
Glutathione-unsaturated fluorotelomer alcohol conjugate (6:2 uFTOH-GSH; DTXSID801348637) (Zhang et al., 2020)

Conjugation: Hydrolysis of glutathione-vinyl ether conjugate to cysteine-vinyl ether conjugate_PTP
SCHEME:

· Glutathione-vinyl ether conjugate (DTXSID10973690) (Jin et al., 1995)

SCHEME:

· 8:2 S-Cys-Glycine-unsaturated fluorotelomer carboxylic (8:2 FTUCA-SCysGly) (Fasano et al., 2009; Zhang et al.,2020)

· 6:2 S-Cys-glycine-unsaturated fluorotelomer carboxylic acid (6:2 FTUCA-SCysGly) (Zhang et al., 2016)

SCHEME:

EXAMPLES:
· S-Cys-Glycine-unsaturated fluorotelomer alcohol conjugate (6:2 uFTOH-SCysGly; DTXSID501348638) (Fasano et al., 2009; Zhang et al., 2020)

Conjugation: Hydrolysis of sulfide conjugate to carboxylic acid
SCHEME:

EXAMPLES:
· 1,1,3,3,3-pentafluoro-2-(fluoromethoxy)propane-1-thiol (DTXSID101348650) (Iyer & Anders, 1996; Spraklin & Kharasch, 1996)

Conjugation: Hydrolysis of vinyl sulfide conjugate to carboxylic acid
SCHEME:

EXAMPLES:
· (1Z)-1,3,3,3-tetrafluoro-2-(fluoromethoxy)prop-1-ene-1-thiol (DTXSID201348653) (Iyer & Anders, 1996; Spraklin & Kharasch, 1996)

Conjugation: Reduction of alpha-keto glutathione conjugate to alpha-hydroxy glutathione conjugate
SCHEME:

EXAMPLES:
· 2-amino-4-{[1-(carboxymethylcarbamoyl)-2-[(3,3,3-trifluoro-2-oxopropyl)sulfanyl]ethyl]carbamoyl}butanoic acid (Schuster et al., 2010)

Conjugation: Reduction of glutathione-unsaturated fluorotelomer aldehyde conjugate to glutathione-unsaturated fluorotelomer alcohol conjugate
SCHEME:

EXAMPLES:
· 6:2 Glutathione-unsaturated fluorotelomer aldehyde conjugate (6:2 FTUAL-GSH; DTXSID501348664) (Zhang et al., 2020)

· 8:2 Glutathione-unsaturated fluorotelomer aldehyde conjugate (8:2 FTUAL-GSH) (Fasano et al., 2009; Nabb et al., 2007; Zhang et al., 2016)

Conjugation: S-Dealkylation of cysteine-ether conjugate to beta thio ether_PTP
SCHEME:

EXAMPLES:
· 2-amino-3-{[1,1,3,3,3-pentafluoro-2-(fluoromethoxy)propyl]sulfanyl}propanoic acid (DTXSID601348667) (Iyer & Anders 1996)

Conjugation: S-Dealkylation of cysteine-vinyl ether conjugate to beta thio vinyl ether_PTP
SCHEME:

EXAMPLES:
· 2-amino-3-{[(1Z)-1,3,3,3-tetrafluoro-2-(fluoromethoxy)prop-1-en-1-yl]sulfanyl}propanoic acid (DTXSID301348668 ) (Iyer & Anders, 1996)

Conjugation: Sulfate-fluorotelomer alcohol conjugate formation
SCHEME:

EXAMPLES:
· 8:2 Fluorotelomer alcohol (DTXSID7029904) (Butt et al., 2010a; Fasano et al., 2009; Dagnino et al., 2016; Martin et al., 2005; Nabb et al., 2007)

Conjugation: Taurine-fluorotelomer acid conjugate formation
SCHEME:

EXAMPLES:
· 7:3 Fluorotelomer carboxylic acid (7:3 FTCA, DTXSID90382620) (Fasano et al., 2009; Nabb et al., 2007)

Decarboxylation Schemes
Decarboxylation: Alpha hydroxy fluorotelomer carboxylic acid to fluorotelomer aldehyde
SCHEME:

EXAMPLES:
· 2-Hydroxy-4,4,5,5,6,6,7,7,8,8,8-undecafluorooctanoic acid (DTXSID901347244) (Martin et al., 2005)

Decarboxylation: Beta carboxy ketone to methyl ketone
SCHEME:

EXAMPLES:
· 4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-pentadecafluoro-3-oxodecanoic acid (7:3 ϐ-keto Acid; DTXSID001348671) (Butt et al., 2010a; Fasano et al., 2009; Nabb et al., 2007)

Epoxidation Scheme
Epoxidation: Alkene to epoxide_PTP
SCHEME:

This scheme includes an exclude rule to prevent epoxide formation at the carbon-carbon double bond adjacent to the carboxylic acid group in beta fluoro unsaturated telomer acids or the carbon-carbon double bond adjacent to the aldehyde group in beta fluoro unsaturated telomer aldehydes
EXAMPLES:
· 2,3,3,3-Tetrafluoropropene (DTXSID4074728) (Schuster et al., 2010)

Hydrolysis Schemes
Hydrolysis: Acid fluoride to carboxylic acid
SCHEME:

EXAMPLES:
· 2,3,3,3-Tetrafluoropropanoyl fluoride (DTXSID20536674) (Koster et al, 1994)

Hydrolysis: Alpha difluoro alcohol to acid fluoride
SCHEME:

EXAMPLES:
· 1,1,2,3,3,3-Hexafluoropropan-1-ol (DTXSID701348672)(Koster et al., 1994)

Hydrolysis: Alpha fluoro secondary alcohol to ketone
SCHEME:

EXAMPLES:
· 1,2,2,3,3,4,4-heptafluorocyclobutanol (DTXSID401348673) (Andreades and England, 1961)

The product shown in brackets is hexafluorocyclobutanone hydrate, an intermediate proposed by Andreades and England (1961). This molecule is a geminal diol which will be transformed to the carbonyl form shown as the final product.
Hydrolysis: Amide to carboxylic acid
SCHEME:

EXAMPLES:
· Trifluoroacetamide (DTXSID1059868) (Meresaar and Bratt, 1974)

Hydrolysis: Carboxylic acid ester to carboxylic acid
SCHEME:

EXAMPLES:
· 8:2 Fluorotelomer acrylate (8:2 FTAc; DTXSID5067348) (Butt et al., 2010c)

Hydrolysis: Diperfluorophosphinate to perfluorophosphonate
SCHEME:

EXAMPLES:
· Diperfluoro-phosphinate (C6/C6 PFPiA; DTXSID00786953) (Lee et al., 2012)

· Diperfluoro-phosphinate (C8/C6 PFPiA) (Lee et al., 2012)

· Diperfluoro-phosphinate (C8/C8 PFPiA) (Lee et al., 2012)

Hydrolysis: Diphosphate ester to monophosphate ester
SCHEME:

EXAMPLES:
· 8:2 Fluorotelomer phosphate diester (8:2 diPAP; DTXSID90218051) (Cui et al., 2021; Dagnino et al., 2016; Deon and Mabury, 2007)

Hydrolysis: Epoxide to diol_PTP
SCHEME:

EXAMPLES:
· 2-Fluoro-2-(trifluoromethyl) oxirane (DTXSID60871339) (Schuster et al., 2008)

Hydrolysis: Fluorotelomer acid to unsaturated telomer acid
SCHEME:

EXAMPLES:
· 6:2 Fluorotelomer carboxylic acid (6:2 FTA; DTXSID60892569) (Ruan et al., 2014; Zhang et al., 2020)

· 8:2 Fluorotelomer carboxylic acid (8:2 FTCA) (Butt et al., 2010a; Fasano et al., 2009; Martin et al., 2005; Nabb et al., 2007; Zhang et al., 2016)

Hydrolysis: Fluorotelomer aldehyde to fluorotelomer unsaturated aldehyde
SCHEME:

EXAMPLES:
· 8:2 Fluorotelomer aldehyde (8:2 FTAL; DTXSID10895489) (Butt et al., 2010a; Fasano et al., 2009; Martin et al., 2005; Nabb et al., 2007; Zhang et al., 2016)

Hydrolysis: Fluorotelomer iodide to fluorotelomer alcohol
SCHEME:

EXAMPLES:
· 6:2 Fluorotelomer iodide (6:2 FTI; DTXSID2047565) (Ruan et al., 2014)

Hydrolysis: Monophosphate ester to alcohol
SCHEME:

EXAMPLES:
· 8:2 Fluorotelomer phosphate monoester (DTXSID60874027) (Cui et al., 2021; Dagnino et al., 2016; Deon and Mabury, 2007)

Hydrolysis: Perfluorinated epoxide to alpha-keto carboxylic acid
SCHEME:

EXAMPLES:
· Hexafluoropropene oxide (HFPO, DTXSID6029177) (Kutsuna, 2018)

Kutsana (2018) shows the formation of numerous possible intermediates in the proposed reaction pathway for the hydrolysis of HFPO; however, the “Hydrolysis: Perfluorinated epoxide to alpha keto carboxylic acid” scheme is written to capture only the overall transformation of HFPO to the final product (3,3,3‐trifluoro‐2‐oxopropanoic acid).
Hydrolysis: Sulfonamide to sulfonic acid
SCHEME:

EXAMPLES:
· Perfluorooctane sulfonamide (FOSA; DTXSID3038939) (Peng et al., 2014; Zhao et al., 2018)

Hydrolysis: Sulfonyl fluoride to sulfonic acid
SCHEME:

EXAMPLES:
· 1‐chloro‐1,2,2,2‐tetrafluoroethanesulfonyl fluoride (Knunyants and Sokols,1972)

Hydroxylation Schemes
Hydroxylation: Fluorotelomer acid to alpha-hydroxy fluorotelomer acid_PTP
SCHEME:

EXAMPLES:
· 8:2 Fluorotelomer carboxylic acid (8:2 FTCA; DTXSID50451109; Martin et al., 2005)

Hydroxylation: Unsaturated Fluorotelomer acid to beta-hydroxy unsaturated fluorotelomer acid_PTP
SCHEME:

The beta-hydroxy product in this reaction scheme may be converted to the beta-keto form through tautomerization.
EXAMPLES:
· 8:2 Fluorotelomer unsaturated acid (8:2 FTUA; DTXSID001019143) (Nabb et al., 2007; Fasano et al., 2009)

SCHEME:

The beta-hydroxy product in this reaction scheme may be converted to the beta-keto form through tautomerization.
EXAMPLES:
· 8:2 Fluorotelomer unsaturated aldehyde (8:2 FTUAL; DTXSID701026622) (Nabb et al., 2007; Fasano et al., 2009)

N-Deacetylation Schemes
SCHEME:

EXAMPLES:
· 6:2 fluorotelomer sulfonamide alkylbetaine (6:2 FTAB; DTXSID80860503) (Moe et al., 2012)

SCHEME:

EXAMPLES:
· 6:2 Fluorotelomer sulfonamido propyl methyl amino acetic acid (6:2 FTSA-Pr-MeAA; DTXSID201034672) (Moe et al., 2012)

N-Deacetylation: N-acetyl N-alkyl sulfonamide to N-alkyl sulfonamide
SCHEME:

EXAMPLES:
· N-ethyl perfluorooctane sulfonamido acetate (N-EtFOSAA; DTXSID5062760) (Peng et al., 2014)

N-Deacetylation: N-acetyl sulfonamide to sulfonamide
SCHEME:
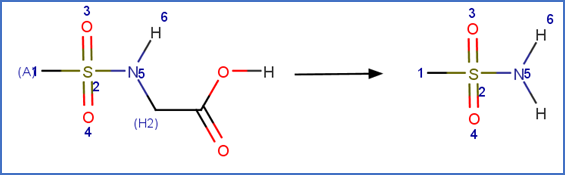
EXAMPLES:
· 2(perfluorooctanesulfonamido)acetic acid (FOSAA; DTXSID40440941) (Zhao et al., 2018)

N-Dealkylation Schemes
N-Dealkylation: N-alkyl sulfonamide to sulfonamide
SCHEME:

EXAMPLES:
· N-Ethylperfluorooctanesulfonamide (N-EtFOSA; DTXSID1032646) (Peng et al., 2014; Tomy et al., 2008; Zhao et al., 2018)

N-Demethylation Schemes
SCHEME:

EXAMPLES:
· 6:2 fluorotelomer sulfonamido propyl dimethyl amine (DTXSID30880433) (Moe et al., 2012)

SCHEME:

EXAMPLES:
· N-(Carboxymethyl)-N,N-dimethyl-3-[(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctane-1-sulfonyl)amino]propan-1-aminium (6:2 FTAB; DTXSID80860503) (Moe et al.,2012)
N-Demethylation: Fluorotelomer sulfonamide N-methyl alkylamine to fluorotelomer sulfonamide alkylamine
SCHEME:

EXAMPLES:
· 6:2 Fluorotelomer sulfonamido propyl methyl amine (DTXSID001034707) (Moe et al., 2012)

O-Demethylation Schemes
O-Demethylation: Alpha-difluoro methyl ether to alcohol
SCHEME:

EXAMPLES:
· 1,1,2,3,3,3-Hexafluoropropyl methyl ether (DTXSID80959148) (Koster et al., 1994)

O-Demethylation: Fluoromethyl ether to secondary alcohol
SCHEME:

EXAMPLES:
· Sevoflurane (DTXSID8046614) (Kharasch et al., 1995)

Oxidation Schemes
SCHEME:

EXAMPLES:
· 7:3 Fluorotelomer unsaturated aldehyde (Fasano et al., 2009; Nabb et al., 2007)

Oxidation: Beta-hydroxy fluorotelomer unsaturated aldehyde to beta-hydroxy fluorotelomer unsaturated acid
SCHEME:

EXAMPLES:
· 7:3 β-hydroxy unsaturated fluorotelomer aldehyde (7:3 β-OH FTUAL) (Fasano et al., 2009; Nabb et al., 2007)

Oxidation: Beta-keto aldehyde to beta-keto acid_PTP
SCHEME:

EXAMPLES:
· 4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-pentadecafluoro-3-oxodecanal (DTXSID701348696) (Butt et al., 2010a; Fasano et al., 2009; Nabb et al., 2007)

Oxidation: Beta oxidation of beta keto fluorotelomer acid_PTP
SCHEME:

EXAMPLES:
· 3-Oxo-4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-pentadecafluorodecanoic acid (7:3 ϐ-keto Acid; DTXSID001348671) (Butt et al., 2010a)

Oxidation: Fluorotelomer alcohol to fluorotelomer aldehyde
SCHEME:

EXAMPLES:
· 6:2 Fluorotelomer alcohol (6:2 FTOH; DTXSID5044572) (Martin et al., 2005; Zhang et al., 2020)

· 8:2 Fluorotelomer alcohol (8:2 FTOH; DTXSID7029904) (Butt et al., 2010a; Fasano et al., 2009; Martin et al., 2005; Nabb et al., 2007; Zhang et al., 2016)

Oxidation: Fluorotelomer alcohol to fluorotelomer carboxylic acid with loss of CF2 and methyl groups
SCHEME:

EXAMPLES:
· 5:2 Secondary fluorotelomer alcohol (5:2 sFTOH; DTXSID80597206) (Zhang et al., 2020)

Oxidation: Fluorotelomer alcohol to fluorotelomer carboxylic acid with loss of methyl group
SCHEME:

EXAMPLES:
· 5:2 Secondary fluorotelomer alcohol (5:2 sFTOH; DTXSID80597206) (Zhang et al., 2020)

· 7:2 Secondary fluorotelomer alcohol (7:2 sFTOH; DTXSID10517598) (Fasano et al., 2009; Zhang et al., 2016)

Oxidation: Fluorotelomer aldehyde to fluorotelomer carboxylic acid
SCHEME:

EXAMPLES:
· 6:2 Fluorotelomer aldehyde (6:2 FTAL; DTXSID20895379) (Martin et al., 2005; Zhang et al., 2020)

· 8:2 Fluorotelomer aldehyde (8:2 FTAL; DTXSID10895489) (Butt et al., 2010a; Fasano et al., 2009; Martin et al., 2005; Nabb et al., 2007; Zhang et al., 2016)

Oxidation: Fluorotelomer carboxylic acid to 2,3-unsaturated fluorotelomer carboxylic acid
SCHEME:

EXAMPLES:
· 5:3 Fluorotelomer carboxylic acid (5:3 FTCA; DTXSID20874028) (Zhang et al., 2020)

Oxidation: Fluorotelomer iodide to alpha-beta unsaturated fluorotelomer iodide_PTP
SCHEME:

EXAMPLES:
· 6:2 Fluorotelomer iodide (6:2 FTI; DTXSID2047565) (Ruan et al., 2014)

Oxidation: Fluorotelomer unsaturated aldehyde to fluorotelomer unsaturated carboxylic acid
SCHEME:

EXAMPLES:
· 8:2 Fluorotelomer unsaturated aldehyde (8:2 FTUAL; DTXSID701026622) (Butt et al., 2010a; Nabb et al., 2007)

Oxidation: N-Alkyl sulfonamide alcohol to N-alkyl sulfonamide carboxylic acid

EXAMPLES:
· N-Ethyl perfluorooctane sulfonamido ethanol (N-EtFOSE; DTXSID6027426) (Peng et al., 2014)

Oxidation: X:3 fluorotelomer aldehyde to X:3 fluorotelomer carboxylic acid
SCHEME:

EXAMPLES:
· 7:3 Fluorotelomer aldehyde (Fasano et al., 2009; Nabb et al., 2007)

Reduction Schemes
Reduction: 2,3-Unsaturated fluorotelomer carboxylic acid to fluorotelomer carboxylic acid
SCHEME:

EXAMPLES:
· 7:3 Fluorotelomer unsaturated carboxylic acid (7:3 FTUCA; DTXSID701026589) (Zhang et al., 2016; Butt et al., 2010a; Fasano et al., 2009; Nabb et al., 2007)

· 5:3 Fluorotelomer unsaturated carboxylic acid (5:3 FTUCA; DTXSID401026580) (Zhang et al., 2020)

Reduction: Beta-F fluorotelomer unsaturated acid to beta-H fluorotelomer unsaturated acid
SCHEME:

EXAMPLES:
· 8:2 Fluorotelomer unsaturated carboxylic acid (8:2 FTUCA; DTXSID60825615) (Zhang et al., 2016; Fasano et al., 2006; Nabb et al., 2007)

Reduction: Beta-F fluorotelomer unsaturated aldehyde to beta-H fluorotelomer unsaturated aldehyde
SCHEME:

EXAMPLES:
· 8:2 Fluorotelomer unsaturated aldehyde (8:2 FTUAL; DTXSID701026622) (Fasano et al., 2009; Nabb et al., 2007)

Reduction: Beta-H fluorotelomer unsaturated aldehyde to fluorotelomer aldehyde
SCHEME:

EXAMPLES:
· 7:3 Fluorotelomer unsaturated aldehyde (7:3 FTUAL) (Fasano et al., 2009; Nabb et al., 2007)

Reduction: Hydrogenolysis of chlorinated perfluorinated ether
SCHEME:

EXAMPLES:
· Chlorinated perfluorinated ether (DTXSID801348699) (Yi et al., 2020)

Reduction: Methyl ketone to alcohol
SCHEME:

EXAMPLES:
· Methyl Pentadecafluoroheptyl Ketone (DTXSID50504109) (Butt et al., 2010a; Fasano et al., 2009; Nabb et al., 2007)

Reduction: Perfluoroalkyl sulfonyl fluoride to perfluoroalkyl sulfinic acid
SCHEME:

EXAMPLES:
· Perfluoroalkyl sulfonyl fluoride (PFBSF; DTXSID20861913) (Jin et al., 2020)

· Perfluoroalkyl sulfonyl fluoride (PFHSF; DTXSID3059973) (Jin et al., 2020)

· Perfluoroalkyl sulfonyl fluoride (PFOSF; DTXSID5027140) (Jin et al., 2020)

Tautomerization Schemes
Tautomerization: Beta hydroxy unsaturated fluorotelomer acid to beta keto fluorotelomer acid
SCHEME:

EXAMPLES:
· 7-3 β-Hydroxy unsaturated acid (7:3 ϐ-OH UA) (Fasano et al., 2009; Nabb et al., 2007)

Tautomerization: Beta hydroxy unsaturated fluorotelomer aldehyde to beta keto fluorotelomer aldehyde
SCHEME:

EXAMPLES:
· 7-3 β-Hydroxy unsaturated aldehyde (7:3 ϐ-OH FTUAL) (Fasano et al., 2009; Nabb et al., 2007)

Rank Levels
|
Rank
|
Upper Limit (days)
|
Upper Limit in Other Units
|
|
7 |
0.020833333 |
30 minutes |
|
6 |
0.138888889 |
200 minutes |
|
5 |
1 |
1 day |
|
4 |
7 |
1 week |
|
3 |
60 |
2 months |
|
2 |
365 |
1 year |
|
1 |
3650 |
10 years |
|
|
|
|
Rank of Individual Reaction Schemes
|
# |
Scheme |
Rank |
|
|
Conjugation |
|
|
1 |
Conjugation: Acetylation of S-Cys-unsaturated fluorotelomer acid conjugate to S-Cys-N-acetyl-unsaturated fluorotelomer acid_PTP |
5 |
|
2 |
Conjugation: Acetylation of S-Cys-unsaturated fluorotelomer acid conjugate to S-Cys-N-acetyl-unsaturated fluorotelomer alcohol_PTP |
5 |
|
3 |
Conjugation: Glucuronide-fluorotelomer alcohol conjugate formation |
5 |
|
4 |
Conjugation: Glucuronide-fluorotelomer sec-alcohol conjugate formation |
5 |
|
5 |
Conjugation: Glutathione-epoxide conjugate formation |
5 |
|
6 |
Conjugation: Glutathione-ether conjugate formation |
6 |
|
7 |
Conjugation: Glutathione-unsaturated fluorotelomer acid conjugate formation |
5 |
|
8 |
Conjugation: Glutathione-unsaturated fluorotelomer aldehyde conjugate formation |
5 |
|
9 |
Conjugation: Glutathione-vinyl ether conjugate formation |
6 |
|
10 |
Conjugation: Hydrolysis of glutathione-ether conjugate to cysteine-ether conjugate |
5 |
|
11 |
Conjugation: Hydrolysis of glutathione-unsaturated fluorotelomer acid conjugate to S-Cys-Gly-unsaturated acid |
5 |
|
12 |
Conjugation: Hydrolysis of glutathione-unsaturated fluorotelomer alcohol conjugate to S-CysGly-unsaturated fluorotelomer alcohol_PTP |
5 |
|
13 |
Conjugation: Hydrolysis of glutathione-vinyl ether conjugate to cysteine-vinyl ether conjugate |
5 |
|
14 |
Conjugation: Hydrolysis of S-Cys-Gly-unsaturated fluorotelomer alcohol conjugate to S-Cys-unsaturated fluorotelomer alcohol_PTP |
5 |
|
15 |
Conjugation: Hydrolysis of S-CysGly-unsaturated fluorotelomer acid conjugate to S-Cys-unsaturated fluorotelomer acid_PTP |
5 |
|
16 |
Conjugation: Hydrolysis of sulfide conjugate to carboxylic acid |
5 |
|
17 |
Conjugation: Hydrolysis of vinyl sulfide conjugate to carboxylic acid |
5 |
|
18 |
Conjugation: Reduction of alpha-keto glutathione conjugate to alpha-hydroxy glutathione conjugate |
5 |
|
19 |
Conjugation: Reduction of Glutathione-unsaturated fluorotelomer aldehyde conjugate to Glutathione-unsaturated fluorotelomer alcohol conjugate |
5 |
|
20 |
Conjugation: S-Dealkylation of cysteine-ether conjugate to beta thio ether_PTP |
4 |
|
21 |
Conjugation: S-Dealkylation of cysteine-vinyl ether conjugate to beta thio vinyl ether_PTP |
4 |
|
22 |
Conjugation: Sulfate-fluorotelomer alcohol conjugate formation |
5 |
|
23 |
Conjugation: Taurine-fluorotelomer acid conjugate formation |
5 |
|
|
Decarboxylation |
|
|
24 |
Decarboxylation: Alpha hydroxy fluorotelomer carboxylic acid to fluorotelomer aldehyde |
4 |
|
25 |
Decarboxylation: Beta carboxy ketone to methyl ketone |
4 |
|
|
Epoxidation |
|
|
26 |
Epoxidation: Alkene to epoxide_PTP |
4 |
|
|
Hydrolysis |
|
|
27 |
Hydrolysis: Acid fluoride to carboxylic acid |
7 |
|
28 |
Hydrolysis: Alpha difluoro alcohol to acid fluoride |
7 |
|
29 |
Hydrolysis: Alpha fluoro secondary alcohol to ketone |
7 |
|
30 |
Hydrolysis: Amide to carboxylic acid |
4 |
|
31 |
Hydrolysis: Carboxylic acid ester to carboxylic acid |
7 |
|
32 |
Hydrolysis: Diperfluoro-phosphinate to monoperfluoro-phosphonate |
4 |
|
33 |
Hydrolysis: Diphosphate ester to monophosphate ester |
4 |
|
34 |
Hydrolysis: Epoxide to diol_PTP |
5 |
|
35 |
Hydrolysis: Fluorotelomer acid to unsaturated telomer acid |
5 |
|
Hydrolysis: Fluorotelomer aldehyde to fluorotelomer unsaturated aldehyde |
7 |
|
|
37 |
Hydrolysis: Fluorotelomer iodide to fluorotelomer alcohol |
5 |
|
38 |
Hydrolysis: Monophosphate ester to alcohol |
4 |
|
39 |
Hydrolysis: Perfluorinated epoxide to alpha keto carboxylic acid |
7 |
|
40 |
Hydrolysis: Sulfonamide to sulfonic acid |
4 |
|
41 |
Hydrolysis: Sulfonyl fluoride to sulfonate |
3 |
|
|
Hydroxylation |
|
|
42 |
Hydroxylation: Fluorotelomer acid to alpha-hydroxy fluorotelomer acid_PTP |
4 |
|
43 |
Hydroxylation: Unsaturated Fluorotelomer acid to beta-hydroxy unsaturated fluorotelomer acid_PTP |
4 |
|
44 |
Hydroxylation: Unsaturated Fluorotelomer aldehyde to beta-hydroxy unsaturated fluorotelomer aldehyde_PTP |
4 |
|
|
N-Deacetylation |
|
|
45 |
N-Deacetylation: Fluorotelomer sulfonamide N-dimethyl N-acetyl betaine to fluorotelomer sulfonamide N-dimethyl alkylamine |
4 |
|
46 |
N-Deacetylation: Fluorotelomer sulfonamide N-methyl N-acetyl alkylamine to fluorotelomer sulfonamide N-methyl alkylamine |
4 |
|
47 |
N-Deacetylation: N-acetyl sulfonamide to sulfonamide |
4 |
|
48 |
N-Deacetylation: N-acetyl, N-alkyl sulfonamide to N-alkyl sulfonamide |
4 |
|
|
N-Dealkylation |
|
|
49 |
N-Dealkylation: N-alkyl sulfonamide to sulfonamide |
4 |
|
|
N-Demethylation |
|
|
50 |
N-Demethylation: Fluorotelomer sulfonamide N-dimethyl alkylamine to fluorotelomer sulfonamide N-methyl alkylamine |
4 |
|
51 |
N-Demethylation: Fluorotelomer sulfonamide N-dimethyl N-acetyl betaine to fluorotelomer sulfonamide N-methyl N-acetyl alkylamine |
4 |
|
52 |
N-Demethylation: Fluorotelomer sulfonamide N-methyl alkylamine to fluorotelomer sulfonamide alkylamine |
4 |
|
|
O-Demethylation |
|
|
53 |
O-Demethylation: Alpha difluoro methyl ether to alcohol |
7 |
|
54 |
O-Demethylation: Fluoromethyl ether to secondary alcohol |
4 |
|
|
Oxidation |
|
|
55 |
Oxidation: Beta-H unsaturated fluorotelomer aldehyde to beta-H unsaturated fluorotelomer carboxylic acid |
4 |
|
56 |
Oxidation: Beta-hydroxy fluorotelomer unsaturated aldehyde to beta-hydroxy fluorotelomer unsaturated acid |
4 |
|
57 |
Oxidation: Beta oxidation of beta keto fluorotelomer acid_PTP |
4 |
|
58 |
Oxidation: Beta-keto aldehyde to beta-keto acid_PTP |
4 |
|
59 |
Oxidation: Fluorotelomer alcohol to fluorotelomer aldehyde |
5 |
|
60 |
Oxidation: Fluorotelomer alcohol to fluorotelomer carboxylic acid with loss of CF2 and methyl groups |
4 |
|
61 |
Oxidation: Fluorotelomer alcohol to fluorotelomer carboxylic acid with loss of methyl group |
4 |
|
62 |
Oxidation: Fluorotelomer aldehyde to fluorotelomer carboxylic acid |
6 |
|
63 |
Oxidation: Fluorotelomer carboxylic acid to 2,3-unsaturated fluorotelomer carboxylic acid |
4 |
|
64 |
Oxidation: Fluorotelomer iodide to alpha beta unsaturated fluorotelomer iodide_PTP |
3 |
|
65 |
Oxidation: Fluorotelomer unsaturated aldehyde to fluorotelomer unsaturated carboxylic acid |
4 |
|
66 |
Oxidation: N-Alkyl sulfonamide alcohol to N-alkyl sulfonamide carboxylic acid |
4 |
|
67 |
Oxidation: X:3 fluorotelomer aldehyde to X:3 fluorotelomer carboxylic acid |
4 |
|
|
Reduction |
|
|
68 |
Reduction: 2,3-Unsaturated fluorotelomer carboxylic acid to fluorotelomer carboxylic acid |
4 |
|
69 |
Reduction: Beta-F fluorotelomer unsaturated acid to beta-H fluorotelomer unsaturated acid |
4 |
|
70 |
Reduction: Beta-F fluorotelomer unsaturated aldehyde to beta-H fluorotelomer unsaturated aldehyde |
4 |
|
71 |
Reduction: Beta-H fluorotelomer unsaturated aldehyde to fluorotelomer aldehyde |
4 |
|
72 |
Reduction: Hydrogenolysis of chlorinated perfluorinated ether |
4 |
|
73 |
Reduction: Methyl ketone to alcohol |
4 |
|
74 |
Reduction: Perfluoroalkyl sulfonyl fluoride to perfluoroalkyl sulfinic acid |
4 |
|
|
Tautomerization |
|
|
75 |
Tautomerization: Beta hydroxy unsaturated fluorotelomer acid to beta keto fluorotelomer acid |
7 |
|
76 |
Tautomerization: Beta hydroxy unsaturated fluorotelomer aldehyde to beta keto fluorotelomer aldehyde |
7 |
The development of version 1.0 of the CTS PFAS Metabolic Reaction Library was described in detail in the following publication:
E.J. Weber, C. Tebes-Stevens, J.W. Washington. 2022. Development of a PFAS reaction library: identifying plausible transformation pathways in environmental and biological systems. Environmental Science: Processes & Impacts, 24, pp. 689–753.
Version 1.1 includes revisions to various reaction schemes and the addition of the following reaction schemes:
· Conjugation: Acetylation of S-Cys-unsaturated fluorotelomer alcohol conjugate to S-Cys-N-Acetyl-unsaturated fluorotelomer alcohol_PTP
· Conjugation: Hydrolysis of S-CysGly-unsaturated fluorotelomer acid conjugate to S-Cys-unsaturated fluorotelomer acid_PTP
· Hydroxylation: Fluorotelomer acid to alpha-hydroxy fluorotelomer acid_PTP
· Hydroxylation: Unsaturated Fluorotelomer acid to beta-hydroxy unsaturated fluorotelomer acid_PTP
· Hydroxylation: Unsaturated fluorotelomer aldehyde to beta-hydroxy unsaturated fluorotelomer aldehyde_PTP
· N-Demethylation: Fluorotelomer sulfonamide N-dimethyl alkylamine to fluorotelomer sulfonamide N-methyl alkylamine
· Oxidation: Beta-H unsaturated fluorotelomer aldehyde to beta-H unsaturated fluorotelomer carboxylic acid
· Oxidation: Beta-hydroxy fluorotelomer unsaturated aldehyde to beta-hydroxy fluorotelomer unsaturated acid
· Oxidation: Fluorotelomer unsaturated aldehyde to fluorotelomer unsaturated carboxylic acid
· Oxidation: X:3 fluorotelomer aldehyde to X:3 fluorotelomer carboxylic acid
· Reduction: Beta-F fluorotelomer unsaturated aldehyde to beta-H fluorotelomer unsaturated aldehyde
· Reduction: Beta-H fluorotelomer unsaturated aldehyde to fluorotelomer aldehyde
· Tautomerization: Beta hydroxy unsaturated fluorotelomer acid to beta keto fluorotelomer acid
· Tautomerization: Beta hydroxy unsaturated fluorotelomer aldehyde to beta keto fluorotelomer aldehyde
Three schemes from the version 1.0 library were deleted, because they are covered by other schemes in the version 1.1 library:
· Hydrolysis: Alpha fluoro primary alcohol to aldehyde
· Hydroxylation: Unsaturated fluorotelomer acid to alpha-hydroxy fluorotelomer acid_PTP
· Hydroxylation: Unsaturated Fluorotelomer acid to beta-hydroxy fluorotelomer acid
· Oxidation: Alpha oxidation of an alpha hydroxy fluorotelomer carboxylic acid to a carboxylic acid
· Oxidation: Beta hydroxy fluorotelomer acid to beta keto fluorotelomer acid_PTP
· Reduction: Hydrogenolysis of chlorinated perfluorinated ether sulfonate
References
Andreades, S., & England, D. C. (1961).
α-HALOALCOHOLS. Journal of the American Chemical Society, 83(22),
4670–4671. https://doi.org/10.1021/ja01483a047
Bello, E. A., & Schwinn, D. A. (1996). Molecular biology and medicine. A
primer for the clinician. Anesthesiology, 85(6), 1462–1478.
https://doi.org/10.1097/00000542-199612000-00029
Bunton, C. A., & Fendler, J. H. (1966). The Hydrolysis of Acetyl Fluoride. The
Journal of Organic Chemistry, 31(7), 2307–2312.
https://doi.org/10.1021/jo01345a053
Butt, C. M., Muir, D. C. G., & Mabury, S. A. (2010a). Elucidating the
pathways of poly- and perfluorinated acid formation in rainbow trout. Environmental
Science & Technology, 44(13), 4973–4980.
https://doi.org/10.1021/es100702a
Butt, C. M., Muir, D. C. G., & Mabury, S. A. (2010b). Biotransformation of
the 8:2 fluorotelomer acrylate in rainbow trout. 1. In vivo dietary exposure. Environmental
Toxicology and Chemistry / SETAC, 29(12), 2726–2735.
https://doi.org/10.1002/etc.349
Butt, C. M., Muir, D. C. G., & Mabury, S. A. (2010c). Biotransformation of
the 8:2 fluorotelomer acrylate in rainbow trout. 2. In vitro incubations with
liver and stomach S9 fractions. Environmental Toxicology and Chemistry /
SETAC, 29(12), 2736–2741. https://doi.org/10.1002/etc.348
Crone, B. C., Speth, T. F., Wahman, D. G., Smith, S. J., Abulikemu, G.,
Kleiner, E. J., & Pressman, J. G. (2019). Occurrence of Per- and
Polyfluoroalkyl Substances (PFAS) in Source Water and Their Treatment in
Drinking Water. Critical Reviews in Environmental Science and Technology,
49(24), 2359–2396. https://doi.org/10.1080/10643389.2019.1614848
Dagnino, S., Strynar, M. J., McMahen, R. L., Lau, C. S., Ball, C.,
Garantziotis, S., Webster, T. F., McClean, M. D., & Lindstrom, A. B.
(2016). Identification of Biomarkers of Exposure to FTOHs and PAPs in Humans
Using a Targeted and Nontargeted Analysis Approach. Environmental Science
& Technology, 50(18), 10216–10225.
https://doi.org/10.1021/acs.est.6b01170
Fasano, W. J., Carpenter, S. C., Gannon, S. A., Snow, T. A., Stadler, J. C.,
Kennedy, G. L., Buck, R. C., Korzeniowski, S. H., Hinderliter, P. M., &
Kemper, R. A. (2006). Absorption, distribution, metabolism, and elimination of
8-2 fluorotelomer alcohol in the rat. Toxicological Sciences: An Official
Journal of the Society of Toxicology, 91(2), 341–355.
https://doi.org/10.1093/toxsci/kfj160
Fasano, W. J., Sweeney, L. M., Mawn, M. P., Nabb, D. L., Szostek, B., Buck, R.
C., & Gargas, M. L. (2009). Kinetics of 8-2 fluorotelomer alcohol and its
metabolites, and liver glutathione status following daily oral dosing for 45
days in male and female rats. Chemico-Biological Interactions, 180(2),
281–295. https://doi.org/10.1016/j.cbi.2009.03.015
Iyer, R. A., & Anders, M. W. (1996). Cysteine conjugate
beta-lyase-dependent biotransformation of the cysteine S-conjugates of the
sevoflurane degradation product compound A in human, nonhuman primate, and rat
kidney cytosol and mitochondria. Anesthesiology, 85(6),
1454–1461. https://doi.org/10.1097/00000542-199612000-00028
Jin, L., Baillie, T. A., Davis, M. R., & Kharasch, E. D. (1995).
Nephrotoxicity of sevoflurane compound A
[fluoromethyl-2,2-difluoro-1-(trifluoromethyl)vinyl ether] in rats: evidence
for glutathione and cysteine conjugate formation and the role of renal cysteine
conjugate beta-lyase. Biochemical and Biophysical Research Communications,
210(2), 498–506. https://doi.org/10.1006/bbrc.1995.1688
Jin, L., Davis, M. R., Kharasch, E. D., Doss, G. A., & Baillie, T. A.
(1996). Identification in rat bile of glutathione conjugates of fluoromethyl
2,2-difluoro-1-(trifluoromethyl)vinyl ether, a nephrotoxic degradate of the
anesthetic agent sevoflurane. Chemical Research in Toxicology, 9(2),
555–561. https://doi.org/10.1021/tx950162m
Jin, Z., He, Q., Luo, H., Pan, Y., Sun, C., & Cai, Z. (2020). The oxidation
of cysteine-containing peptides caused by perfluoroalkane sulfonyl fluorides. Journal
of Hazardous Materials, 385, 121564.
https://doi.org/10.1016/j.jhazmat.2019.121564
Kharasch, E. D., Karol, M. D., Lanni, C., & Sawchuk, R. (1995). Clinical
sevoflurane metabolism and disposition. I. Sevoflurane and metabolite
pharmacokinetics. Anesthesiology, 82(6), 1369–1378.
https://doi.org/10.1097/00000542-199506000-00008
Köster, U., Speerschneider, P., Kerssebaum, R., Wittmann, H., & Dekant, W.
(1994). Role of cytochrome P450 2E1 in the metabolism of
1,1,2,3,3,3-hexafluoropropyl methyl ether. Drug Metabolism and Disposition:
The Biological Fate of Chemicals, 22(5), 667–672.
https://www.ncbi.nlm.nih.gov/pubmed/7835215
Kutsuna, S. (2018). Rate constants and C C bond scission ratios for hydrolysis
of 2,2,3-trifluoro-3-(trifluoromethyl)oxirane determined by means of a
closed-circulation reactor. Journal of Fluorine Chemistry, 211,
109–118. https://doi.org/10.1016/j.jfluchem.2018.04.013
Lasier, P. J., Washington, J. W., Hassan, S. M., & Jenkins, T. M. (2011).
Perfluorinated chemicals in surface waters and sediments from northwest
Georgia, USA, and their bioaccumulation in Lumbriculus variegatus. Environmental
Toxicology and Chemistry / SETAC, 30(10), 2194–2201. https://doi.org/10.1002/etc.622
Lee, H., D’eon, J., & Mabury, S. A. (2010). Biodegradation of
polyfluoroalkyl phosphates as a source of perfluorinated acids to the
environment. Environmental Science & Technology, 44(9),
3305–3310. https://doi.org/10.1021/es9028183
Lee, H., De Silva, A. O., & Mabury, S. A. (2012). Dietary bioaccumulation
of perfluorophosphonates and perfluorophosphinates in juvenile rainbow trout:
evidence of metabolism of perfluorophosphinates. Environmental Science &
Technology, 46(6), 3489–3497. https://doi.org/10.1021/es204533m
Li, W., Dong, Z., Zhang, Y., Zeng, Z., Usman, M., & Liu, W.-B. (2019).
Cu-Catalyzed Arylation/Acyl Migration Cascade Reaction of Enaminones: Access to
N-Fused Polycyclic and 2,3-Disubstituted Indoles. The Journal of Organic Chemistry,
84(12), 7995–8005. https://doi.org/10.1021/acs.joc.9b00866
Lin, Y., Ruan, T., Liu, A., & Jiang, G. (2017). Identification of Novel
Hydrogen-Substituted Polyfluoroalkyl Ether Sulfonates in Environmental Matrices
near Metal-Plating Facilities. Environmental Science & Technology, 51(20),
11588–11596. https://doi.org/10.1021/acs.est.7b02961
Martin, J. W., Mabury, S. A., & O’Brien, P. J. (2005). Metabolic products
and pathways of fluorotelomer alcohols in isolated rat hepatocytes. Chemico-Biological
Interactions, 155(3), 165–180.
https://doi.org/10.1016/j.cbi.2005.06.007
Meresaar, U., Bratt, L., Alakuijala, P., & Oinonen, L. (1974). Hydrolysis
of amides. Alkaline and general acid catalyzed alkaline hydrolysis of some
substituted acetamides and benzamides. Acta Chemica Scandinavica, 28a,
715–722. https://doi.org/10.3891/acta.chem.scand.28a-0715
Nabb, D. L., Szostek, B., Himmelstein, M. W., Mawn, M. P., Gargas, M. L.,
Sweeney, L. M., Stadler, J. C., Buck, R. C., & Fasano, W. J. (2007). In
vitro metabolism of 8-2 fluorotelomer alcohol: interspecies comparisons and
metabolic pathway refinement. Toxicological Sciences: An Official Journal of
the Society of Toxicology, 100(2), 333–344.
https://doi.org/10.1093/toxsci/kfm230
Peng, H., Zhang, S., Sun, J., Zhang, Z., Giesy, J. P., & Hu, J. (2014).
Isomer-specific accumulation of perfluorooctanesulfonate from (N-ethyl
perfluorooctanesulfonamido)ethanol-based phosphate diester in Japanese Medaka
(Oryzias latipes). Environmental Science & Technology, 48(2),
1058–1066. https://doi.org/10.1021/es404867w
Royer, L. A., Lee, L. S., Russell, M. H., Nies, L. F., & Turco, R. F.
(2015). Microbial transformation of 8:2 fluorotelomer acrylate and methacrylate
in aerobic soils. Chemosphere, 129, 54–61. https://doi.org/10.1016/j.chemosphere.2014.09.077
Ruan, T., Sulecki, L. M., Wolstenholme, B. W., Jiang, G., Wang, N., & Buck,
R. C. (2014). 6:2 Fluorotelomer iodide in vitro metabolism by rat liver
microsomes: comparison with [1,2-(14)C] 6:2 fluorotelomer alcohol. Chemosphere,
112, 34–41. https://doi.org/10.1016/j.chemosphere.2014.02.068
Schuster, P., Bertermann, R., Rusch, G. M., & Dekant, W. (2010).
Biotransformation of 2,3,3,3-tetrafluoropropene (HFO-1234yf) in rabbits. Toxicology
and Applied Pharmacology, 244(3), 247–253.
https://doi.org/10.1016/j.taap.2009.12.022
Spracklin, D. K., & Kharasch, E. D. (1996). Evidence for metabolism of
fluoromethyl 2,2-difluoro-1-(trifluoromethyl)vinyl ether (compound A), a
sevoflurane degradation product, by cysteine conjugate beta-lyase. Chemical
Research in Toxicology, 9(4), 696–702.
https://doi.org/10.1021/tx9502103
Tomy, G. T., Tittlemier, S. A., Palace, V. P., Budakowski, W. R., Braekevelt,
E., Brinkworth, L., & Friesen, K. (2004). Biotransformation of N-ethyl
perfluorooctanesulfonamide by rainbow trout (Onchorhynchus mykiss) liver
microsomes. Environmental Science & Technology, 38(3),
758–762. https://doi.org/10.1021/es034550j
Washington, J. W., Jenkins, T. M., & Weber, E. J. (2015). Identification of
Unsaturated and 2H Polyfluorocarboxylate Homologous Series and Their Detection
in Environmental Samples and as Polymer Degradation Products. Environmental
Science & Technology, 49(22), 13256–13263.
https://doi.org/10.1021/acs.est.5b03379
Yi, S., Zhu, L., & Mabury, S. A. (2020). First Report on In Vivo
Pharmacokinetics and Biotransformation of Chlorinated Polyfluoroalkyl Ether
Sulfonates in Rainbow Trout. Environmental Science & Technology, 54(1),
345–354. https://doi.org/10.1021/acs.est.9b05258
Zhang, H., Wen, B., Hu, X., Wu, Y., Pan, Y., Huang, H., Liu, L., & Zhang,
S. (2016). Uptake, Translocation, and Metabolism of 8:2 Fluorotelomer Alcohol
in Soybean (Glycine max L. Merrill). Environmental Science & Technology,
50(24), 13309–13317. https://doi.org/10.1021/acs.est.6b03734
Zhang, H., Wen, B., Huang, H., Wang, S., Cai, Z., & Zhang, S. (2020).
Biotransformation of 6:2 fluorotelomer alcohol by the whole soybean (Glycine
max L. Merrill) seedlings. Environmental Pollution , 257, 113513.
https://doi.org/10.1016/j.envpol.2019.113513
Zhao, L., Folsom, P. W., Wolstenholme, B. W., Sun, H., Wang, N., & Buck, R.
C. (2013). 6:2 fluorotelomer alcohol biotransformation in an aerobic river
sediment system. Chemosphere, 90(2), 203–209.
https://doi.org/10.1016/j.chemosphere.2012.06.035
Zhao, S., Wang, B., Zhu, L., Liang, T., Chen, M., Yang, L., Lv, J., & Liu,
L. (2018). Uptake, elimination and biotransformation of N-ethyl perfluorooctane
sulfonamide (N-EtFOSA) by the earthworms (Eisenia fetida) after in vivo and in
vitro exposure. Environmental Pollution , 241, 19–25.
https://doi.org/10.1016/j.envpol.2018.05.046
